Regulatory T cells (thymically and peripherally derived Treg) have critical roles in maintaining immune homeostasis and controlling aberrant/excessive responses to self and non-self antigens. Typically defined as CD4 T cells with high expression of CD25 and FoxP3, these cells mediate tolerance to autoantigens, microflora and food antigens. While there is considerable interest in harnessing Treg activity therapeutically in autoimmune and tissue transplant settings, their activity has garnered significant interest in the immuno-oncology field.
Learn more about our Treg Suppression model
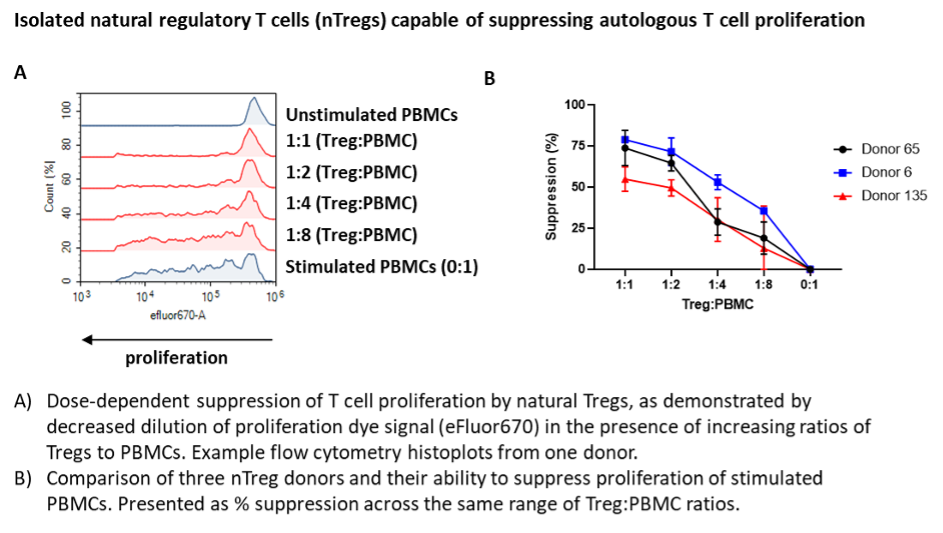
In our whitepaper, we describe how scientists from Antibody Analytics have established a complete Treg suppression assay workflow. The assay includes isolation of natural Treg cells from peripheral blood mononuclear cells (PBMCs) and co-culture with anti-CD3 stimulated autologous PBMCs at different Treg to PBMC ratios to demonstrate functional suppression of the responder (conventional or Tconv) T cell population. This is coupled with Treg immunophenotyping, cytokine release and Treg-specific reversal of suppressive activity with a novel immunotherapy.
Why Tregs?
Tregs are enriched in the tumour microenvironment (TME) and in this context are tumour-permissive, facilitating immune evasion and tumour progression. Tregs are able to suppress conventional CD4 and CD8 T cell responses preventing their efficient targeting of cancer cells, mediating their suppressive function by a variety of mechanisms including: secretion of inhibitory cytokines (e.g. TGFβ, IL-10 and IL-35); suppression by metabolic interference (e.g. by sequestration of IL-2 by means of high CD25 expression or by inhibition via the adenosine pathway); suppression by cytolysis (e.g. granzyme A/B production); and modulation of dendritic cell (DC) function (e.g. via CTLA-4/ CD80/CD86 or LAG3/MHC Class II interactions with DCs) (Figure 1).
These mechanisms are multi-faceted, temporally and spatially, impacting both priming and effector T cell responses in lymphoid and TME settings respectively, in addition to the conditioning of antigen presenting cells (APCs) and innate immune cells to dampen anti-tumour activity.

Tregs as a potential way to fight cancer
Preclinical studies demonstrating that disruption of intra-tumoural Treg activity is associated with reduced tumour burden, and approval of ipilimumab (Yervoy, an anti-CTLA-4 antibody) for treatment of metastatic melanoma, set a clear precedent in targeting this cell population. Accordingly, Tregs and their activity have become attractive candidates in developing novel cancer therapies.
Several therapeutic strategies are now underway to identify different ways in which Tregs may be leveraged for treatment of various tumour types. Numerous Treg targets and approaches are under investigation; CTLA-4, CD25, FoxP3, ubiquitin, GITR, CD39, CCR4, CCR8, IL-10 and TGFβ to name but a few. Consequently, now, more than ever, there is a demand for defined, robust, in vitro Treg assays to test candidate immunotherapies for their ability to modulate Treg phenotype and importantly, functional activity.




